Rydberg Laboratory
The Rydberg Laboratory is a multidisciplinary laboratory and a well-established innovation and collaboration arena at the University. Education and experimental research are conducted in a number of smaller labs within environmental technology, energy, materials, measurement technology, surface metrology and tribology.

The Rydberg Laboratory is a stimulating research environment where innovative intra- and inter-disciplinary research projects are carried out. The Rydberg Laboratory is also a resource used by many of the courses at the university and provides an opportunity for undergraduates and masters students to experience practical research as part of their education.
The Rydberg Laboratory welcomes further collaboration with external institutions and businesses.
The Rydberg Laboratory is run in collaboration between the School of Business, Innovation and Sustainability and the School of Information Technology.
A lab with a history
The Rydberg Laboratory was opened in 2004. It is named after the famous Swedish physicist and mathematician Johannes “Janne” Rydberg, who was born in Halmstad in 1854.
Research units at the Rydberg Laboratory
The Rydberg Laboratory is made up of several smaller lab units. They all conduct a mixture of basic and applied research, often in collaboration with partners from industry and the wider society. Collaborative partners working in applied sciences enjoy access to state-of-the-art equipment and to competent research expertise, all as part of an innovative environment setting.
Environmental Biology Lab
In the research laboratories for biology and environmental science a wide range of biological and chemical analyses can be performed, including analysis of nutrient contents in soils, tissues and water, as well as gas composition and microscopy. The lab also includes an experimental wetland facility for testing the design and function of wetlands, and a biogas test lab for assessing substrates for biogas production.
Fab Lab
The Fab Lab is the digital fabrication and research facility of the Rydberg Laboratory. It houses 3D printers for additive manufacturing of prototypes and products in different polymers; 3D scanners for digitalisation of artefacts; laser and vinyl cutters for prototype manufacturing; textile embroidery machines for soft material design; electronics equipment for smart products design; and a foundry for wax modelling and casting of metal parts. The Fab Lab is part of a world-wide network of more than 1,200 similar labs originating from MIT in Boston. In Fab Lab, students and researchers from academia and industry have regular opportunities to work together with a broader public and experience modern digital manufacturing.
Human Movement Lab
The Human Movement Lab is a research and teaching facility for the study of the physiology and biomechanics of exercise. The focus is on measurements of exercise capacity and movement mechanics, using advanced equipment to measure endurance, strength, power, flexibility, anthropometrics, muscle activation, vibration, kinematics and kinetics.
Mechanics Lab
In the Mechanics Lab students and researchers can study human and machine motion. It is equipped with a 3D-motion analysis system including six cameras and force plates able to measure up to 10 kN. Low-speed and high-speed accelerometers are also available to assess vibrations in applications that include the use of tools or vehicles. The Mechanics Lab also has equipment for tensile and compressive testing to measure material properties such as ultimate tensile strength, breaking strength, and elongation.
Microscopy Lab
The Microscopy Lab houses advanced computerised optical microscopes, a versatile scanning-probe microscope and a scanning electron microscope with element analysis capability for in-depth characterization of materials on different length scales down to atomic resolution.
Optoelectronics Lab
The Optoelectronics Lab houses state-of-the-art equipment for extremely sensitive electrical and optical device characterization. The optical characterization capabilities include a broadband Fourier transform spectrometer with integrated cryostat for measurements down to 5K (-268°C). The electrical characterization units offer nanovolt (10-9V) and femtoampere (10-15A) detection limits. For contacting of samples, the lab is equipped with a modern wedge bonder.
Surface Metrology Lab
The Surface Metrology Lab comprises 2D- and 3D-surface profilers (stylus, optical interferometry and atomic force microscopy) for surface texture and roughness analysis at lengthscales ranging from nanometers to centimeters. The lab is also equipped for measurements of surface properties, including chemical properties using the contact angle method; surface colour and gloss using spectrophotometry; and surface friction.
Tribology Lab
The Tribology Lab houses two tribometers for studying friction, wear and surface damage. A high-load tribometer (up to 20 kN) and a low-load tribometer (up to 1 kN) enable testing during reciprocating and rotary motions of the samples. Test parameters such as temperature, speed, displacement, load, and lubricants flow can be accurately controlled, and data such as friction forces, electrical resistance, and acoustic emissions can be recorded at high frequencies.
Meet Janne Rydberg
The Rydberg Laboratory is named after the mathematician and physicist Johannes “Janne” Rydberg (1852–1919). He is mainly known for devising the famous Rydberg formula which is used to predict the wavelengths of the spectral lines of the elements. He is often referred to as the father of modern atomic spectroscopy.
The mathematician and physicist Johannes Robert (Janne) Rydberg was born in Halmstad in 1854. After final exams from Halmstads Högre Läroverk, he began studies at Lund University in 1873. Rydberg was appointed as assistant in physics at Lund University as early as 1876, although his interests at this stage were dominated by mathematics. Rydberg’s talent in mathematics led to his PhD on the construction of conical sections in 1879. After a second thesis on functional analysis in 1880, Rydberg was appointed as associate professor of mathematics.

Photographer: Per Bagge. Reproduction: Lund University Library.
Rydberg's first article in physics was entitled: Studies on friction electricity, a study that led to his appointment as associate professor of physics at Lund University in 1882. His great interest in numerical patterns and regularities proved highly useful in his studies of the periodic table of the elements. Pioneering work carried out by Mendeleev showed a remarkable periodicity in the chemical and physical properties of the elements when they were arranged according to increasing atomic weight. The connection between the observed spectra of elements and their chemical properties had been studied previously in experiments by Meyer, Kirchhoff and Bunsen, but a theoretical explanation for their experimental findings had not been found. Rydberg pointed out that the periodic table of the elements clearly indicated that inter-atomic forces varied in accordance with the atomic weight, and that many chemical and physical properties derive from recurrent features in the structure of the atoms. He also argued that spectral analyses was the best tool for studying the structure of atoms and was therefore the best tool for unravelling the physics behind the periodic table of the elements. It was not until the advent of quantum mechanics in the 1920s that it was clarified that similarities in the chemical and physical properties of the elements reflect similarities in the valence shell of the atoms.
Due to the ever-increasing sophistication of experimental tools, accurate spectra were already accessible for many elements. The presence of line spectra had previously been noticed by Liveing and Dewar. In the famous article "On the structure of line spectra of the chemical elements" from 1890, Rydberg could describe all known spectral series with the formula:
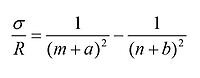
Here σ is the wave number (reciprocal wavelength), R the so-called Rydberg constant, and m and n are integers, with n>m. Of these integers m has a definite value, while n may vary. The numbers a and b are known as quantum defects. It should be mentioned that Balmer a few years before had discovered a mathematical expression that described the spectral lines of hydrogen. His expression is a special case of the Rydberg formula, with m=2 and a=b=0.
In spite of the fact that Rydberg never found a deeper explanation for his formula (this was to be achieved by Niels Bohr), his contributions to modern physics can hardly be overestimated. For this reason Rydberg is often referred to as the father of modern atomic spectroscopy. Rydberg was appointed professor of physics at Lund University in 1901, a position that he retained until his death in 1919.
Research Facilities
The research facilities available within the Rydberg Laboratory are subdivided into the following laboratories:
- Environmental Biology Lab
- Fab Lab
- Human Movement Lab
- Mechanics Lab
- Microscopy Lab
- Optoelectronics Lab
- Surface Metrology Lab
- Tribology Lab
The Rydberg Laboratory houses advanced equipment, including:
- C/N analyser
- Gas chromatographs
- Computerized optical microscope
- Scanning electron microscope with EDS
- Scanning probe microscopes
- Optical profilometers
- Fourier transform spectrometer with integrated low-temperature cryostat
- Low-level current, voltage, capacitance and inductance measurement units
- Tribometers
- Laser cutter
- Laser scanner
- 3D printers

